Analytical Chemistry
Jonathon Duay, Janine Elliott, Jason B. Shear, and Keith J. Stevenson
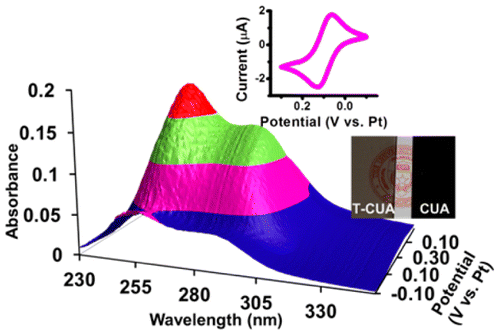
Opaque and transparent carbon ultramicro- to nanoelectrode arrays were made using a previously reported facile versatile fabrication method (Duay et al. Anal. Chem. 2014, 86, 11528). First, opaque carbon ultramicroelectrode arrays (CUAs) were characterized for their analytical response to hydrogen peroxide (H2O2) oxidation using cyclic voltammetry. The alumina blocking layer was found to contribute to the noise and thus had undesirable effects on the array’s limit of detection (LOD) for H2O2 at fast scan rates. Nonetheless, at slower scan rates (ν ≤ 250 mV s–1), the LODs for H2O2 for both opaque (O-CUAs) and transparent arrays (T-CUAs) were found to be lower than previously reported levels for array-based UMEs. LODs as low as 35 nM H2O2 are obtained for T-CUA at a 2.5 mV s–1 scan rate. Furthermore, the transparent arrays were analyzed for their spectroelectrochemical response during the oxidation/reduction of ferrocenemethanol. Results showed very good correlation between the optical and electrochemical response for ferrocenemethanol at a UV wavelength of 254 nm. Thus, these electrodes allow for the in situ mechanistic and kinetic characterization of heterogeneous electrochemical and intermediate homogeneous chemical reactions with high electroanalytical sensitivity, low detection limits, and wide dynamic range.